Recently, a research article entitled "Reprogramming the enzymatic assembly line for site-specific fucosylation" was published in Nature Catalysis by Professor Cao Hongzhi's group. Professor Cao from Shandong University is the corresponding author and Ph.D. student Ye Jinfeng is the first author of this paper. Prof. Chi Lianli, Prof. Xiao Min, Prof. Wang Fengshan from National Glycoengineering Research Center and Professor Wang Ping from Shanghai Jiao Tong University have also made important contributions to this study.

Lewis antigens belong to the family of fucosylated carbohydrate determinants or epitopes. They are common components of various cell surface glycoconjugates and secreted unconjugated glycans, which play pivotal roles in many physiological and pathological processes. Biosynthesis of Lewis antigens involves multiple fucosyltransferases which catalyze the fucosylation of poly-LacNAc carbohydrate backbone in a non-site-specific manner, thus generating a mixture of heterogeneous and incomplete fucosylated Lewis antigen regioisomers. In this study, taking advantage of the substrate specificities of bacteriala2,6-sialyltransferase anda1,3/4-fucosylatransferases, ana2,6-sialylation module was used to introducea2,6-linked sialic acid to specific sites to protect them from fucosylation, thus precisely controlling of enzymatic fucosylation of poly-LacNAc glycans was achieved. The sialic acids can be easily removed by sialidase after fucosylation to provide a variety of fucosylated poly-LacNAc glycans with defined fucosylation patterns. The general applicability and robustness of this reprogramed enzymatic assembly line was exemplified in the synthesis of 22 complex Lewis antigens and chimeric histo blood group antigens with a total of 10 enzyme modules for construction of 10 different glycosidic linkages. This work provides standard compounds for the characterization and quantification of complex natural fucosylated oligosaccharides, and makes it possible to systematically study the biological functions of complex glycan families.
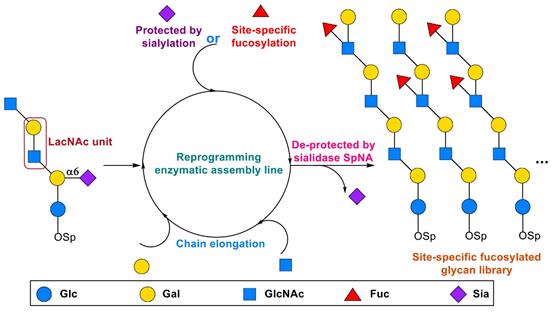
The study of Prof. Cao Hongzhi's group mainly focus on the development of efficient and transferable methods for the systematic synthesis of complex human glycan libraries. Using more than 20 delicately designed enzymatic assembly modules, a series of complex human glycan libraries have been constructed, including tumor-associated carbohydrate antigens, human milk oligosaccharides, blood group antigens and O-mannose glycans. In order to overcome the limitation of substrate applicability and accessibility of glycosyltransferases, Prof. Cao's group introduced various concepts and methods from organic synthesis to enzymatic modular assembly of complex glycans. The controlled synthesis of sialic acid-containing glycans was realized for the first time(J. Am. Chem. Soc. 2014, 136, 5205; J. Am. Chem. Soc. 2019, 141, 4547). In current paper, the site-specific fucosylation of complex glycans have been accomplished, which is a major breakthrough in the field of oligosaccharide synthesis. In recent years, Prof. Cao's group have published a series of papers inJ . Am. Chem. Soc., Angew. Chem. Int. Ed., ACS Catal, et al. This work was supported by the National Natural Science Foundation of China, the open project of the State key Laboratory of Microbial Technology and "Innovative Team of Pharmaceutical Chemical Biology of Shandong University".
Paper link:https://www.nature.com/articles/s41929-019-0281-z
Homepage:https://caolab.wixsite.com/hongzhicao
Source: National Glycoengineering Research Center
Written by: Ye Jinfeng
Edited by: Xie Tingting