Recently, Prof. Dang Feng’ group reported a serious works for the 2D materials and oxides as the high performance cathode catalysts in Li-O2batteries. Due to their superior energy density, lithium-oxygen batteries (LOBs) have recently received tremendous attention for use in large-scale energy storage and next-generation electric or hybrid electric vehicles, which could deliver a theoretical energy density of 3623 Wh kg−1. Unfortunately, some fundamental hurdles still limit the battery’s practical utilization including high overpotentials, poor cycling performance, low capacity far from theoretical value, and low round-trip efficiency. These critical issues are closely connected to several technological barriers, such as sluggish redox kinetics, chemical instabilities of reaction intermediates, parasitic reactions between carbon and electrolyte/solid products, and inferior electronic/ionic conductivity of inactive Li2O2products. The reversible formation and decomposition of battery products Li2O2on the cathode catalyst determine the electrochemical performance to a great extent.
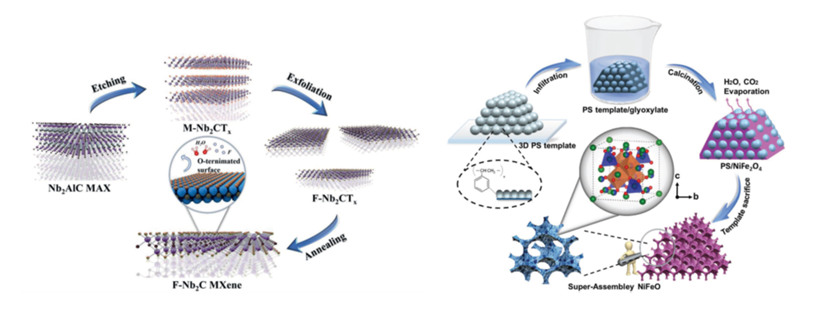
Fig. 1 Synthesis process of Nb2C MXene and NiFeO spinel catalysts for LOBs
MXenes are predicted to be one of the most impressive 2D materials for energy applications. Thehigh catalytic activity and unique reaction kineticsof Nb2C MXene with a uniform O-terminated surface as a cathode material for LOBs is firstly demonstratedby the research group (Adv. Energy Mater.2021, 11, 2002721);For the Ti2C MXene with a inhomogeneous surface condition, our research revealed a polarized nucleation and growth reaction kinetics of discharge products to form the porous structure from spatial-direction accumulated nanoflakes, which can provide an efficient pathway for mass transfer(Energy Storage Mater.2021, 35, 669). For the spinel structured NiFeO, it is revealed that the catalytic capability is attributed to thesynergistic effect of surface condition and inside ion occupation. The octahedron predominant spinel provides a stable polycrystal structure and optimized electronic structure, which dominates the discharge/charge products evolution with multiformation kinetics of crystal Li2O2and Li2−xO2at low and high rate conditions and energetically favors the mass transport between the electrode/electrolyte interfaces(Adv. Energy Mater.2020, 10, 1904262). Futhermore, the superior catalytic capability of CeO2nanocrystals derived from lattice matching with Li2O2, controlled high active surface and functionl micro-structure was identified (Adv. Energy Mater.2019, 9, 1901751). Moreover, the effect of oxygen vaccancy during the ORR/OER process was clarified (J. Mater. Chem. A, 2019, 7, 6552). Appling the lattice matching between CeO2and Li2O2, the formation reaction route of discharge products was successfully realized through a CeO2/Co3O4composite catalyst (Applied Materials Today,2020, 19, 100603). Despite the 2D materials and oxide based catalysts, the research group also investigated the catalytic capability of Co-N-C single atom(Eng. Sci.2020, 10, 85)and surface activated MoSi2catalyst(J. Mater. Chem. A2020, 8, 259).
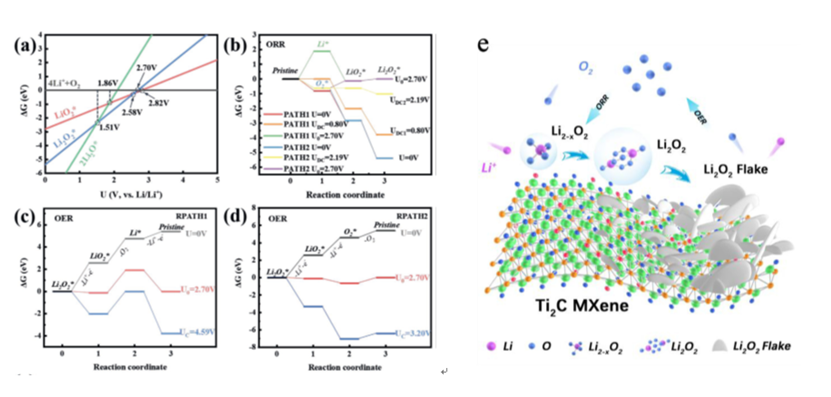
Fig. 2 Theoretical calculation for MXene based catalysts in LOBs
Written by:Dang Feng