Recently, Professor Yu Xiao/Sun Jinpeng's research group, from School of Basic Meidical Sciences, Shandong University, has made new progress in the regulation of adrenal secretion by phosphatase. Their research results, entitled "PTP-MEG2 regulates quantal size and fusion pore opening through two distinct structural bases and substrates" was published on EMBO Reports (The five-year impact factor is 9.214).
Secretion via vesicle exocytosis is a fundamental biological event involved in almost all physiological processes, such as synaptic transmission, immune response and glucose homeostasis. The research on the mechanism of cellular vesicle transport and regulation won the Nobel Prize in Physiology in 2013, but the role of phosphorylation regulation in vesicle secretion is not fully understood. Phosphorylation mainly consists of two types, serine/threonine phosphorylation and tyrosine phosphorylation, which are co-regulated by kinases and phosphatases. The involvement of serine/threonine phosphorylation in vesicle secretion during exocytosis has been partially demonstrated, but the precise role of tyrosine phosphorylation in vesicle secretion and which tyrosine phosphatases are involved in the regulation of vesicle secretion are still poorly understood.
We demonstrate for the first time that tyrosine phosphatase PTP-MEG2 regulates two important steps of the catecholamines secretion by adrenal chromaffin cells,the vesicle fusion process and the formation and dissociation of fusion pores on the membrane, through dephosphorylation of different protein substrates. PTP-MEG2 regulates vesicle fusion through dephosphorylation of NSF. At the same time, using proteomics techniques, bioinformatics prediction and electrochemical detection techniques, the team found that PTP-MEG2 can also regulate the formation and dissociation of fusion pores on the membrane by dephosphorylation of MUNC18-1 and DYNAMIN2. The team further explained the key sites and structural basis of PTP-MEG2 regulated different substrates through biochemical and crystallographic analysis, and accidentally found that PTP-MEG2 can regulate the above two processes through different structural bases (as shown in the figure below). Therefore, specific inhibitors of PTP-MEG2 regulating different secretion processes can be designed according to these structural bases and key sites. This study for the first time systematically elaborated the physiological role and molecular mechanism of PTP-MEG2 in pheochromocyte secretion, found the key regulation and precise mechanism of tyrosine phosphorylation in vesicle secretion, indicated that dynamic tyrosine phosphorylation is an important factor in regulating hormone secretion, and suggested the potential pharmacological value of targeting PTP-MEG2 in the secretion process.
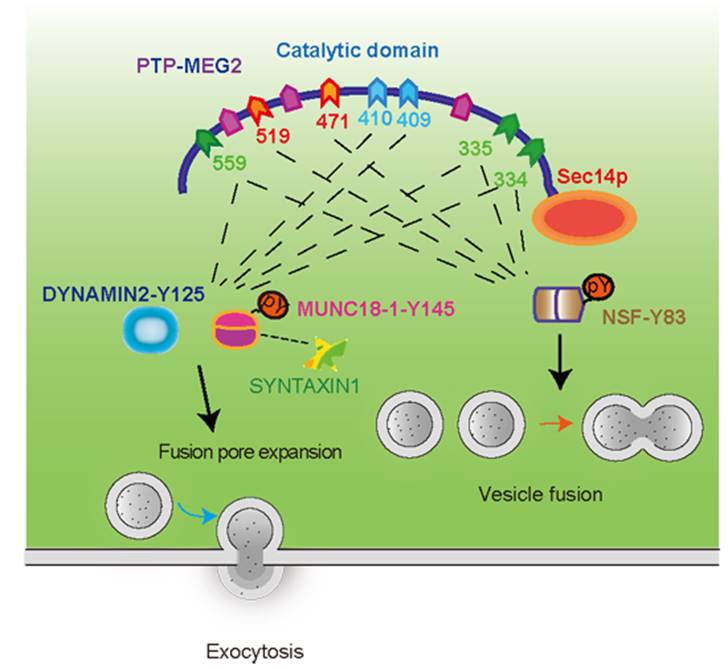
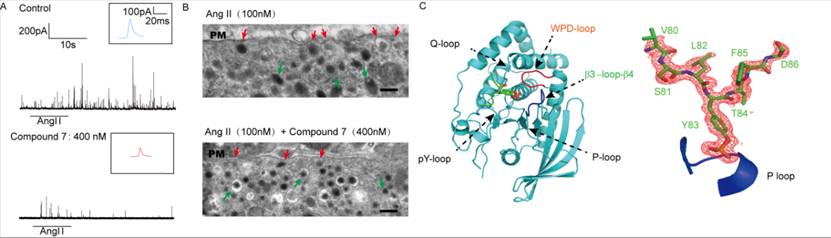
The formation and opening of the fusion pores between vesicles and cell membranes has always been a hot and difficult issue in vesicle secretion. In this paper, the researchers combined many biophysical and electrochemical methods like electron microscopy detection, carbon fiber electrode detection and analysis of the complex crystal structure (below), firstly demonstrated that PTP-MEG2 regulates the initial opening of the fusion pore during exocytosis by interacting with two substrates, MUNC18-1-pY145 and DYNAMIN2-pY125. They demonstrated the role of tyrosine phosphorylation in the formation and opening of fusion pores, revealed the unique structural basis of the process mediated by PTP-MEG2, and how PTP-MEG2 mediated dephosphorylation regulate fusion pore dynamics. Abnormal vesicle secretion has been reported in a variety of diseases, including epilepsy. In view of the multilevel and critical regulatory role of PTP-MEG2 in cell secretion, and the fact that PTP-MEG2 has specific inhibitors with high membrane permeability, clarifying the role of PTP-MEG2 in the release of vesicle fusion is helpful to accelerate translational medicine research targeting PTP-MEG2.
This work was also supported and collaborated by Prof. Zhang Zhongyin of Purdue University, Prof. Ji Zhiliang of Xiamen University, Prof. Wang Changhe of Xi’an Jiaotong University, Prof. Zhao Weidong of Chinese Medical Sciences University, senior engineer Zhu Zhongliang of China Science and Technology University, Prof. Xu Zhigang of Shandong University.
Professor Yu Xiao/Sun Jinpeng's research group, from School of Basic Medical Sciences, Shandong University, focus on the physiological and pathophysiological mechanisms of islet and adrenal metabolism. In previous studies, they have elucidated the role of metabolic homeostasis in islet loop homeostasis (Nature 2020) (J Clin Invest. 2017) (Diabetologia. 2014) (Diabetologia. 2015), accurate regulation mechanisms of islet function by GPCR-related pathways (Br J Pharmacol. 2015), and adhesion receptors regulation to water and salt metabolism in adrenal homeostasis (Elife. 2018). The above researches were supported by the National Natural Science Foundation of China.
The link of this paper: http://doi.org/10.15252/embr.202052141